Traceability has a key role in the monitoring, supervision, and improvement of the overall quality of medical devices and in-vitro devices.
Track your Medical Device up to your end customer
Traceability has a key role in the monitoring, supervision, and improvement of the overall quality of medical devices and in-vitro devices
Serialization, by assigning a unique serial number to a medical device despite its manufacturing origin around the world, is the first step for a traceability solution.
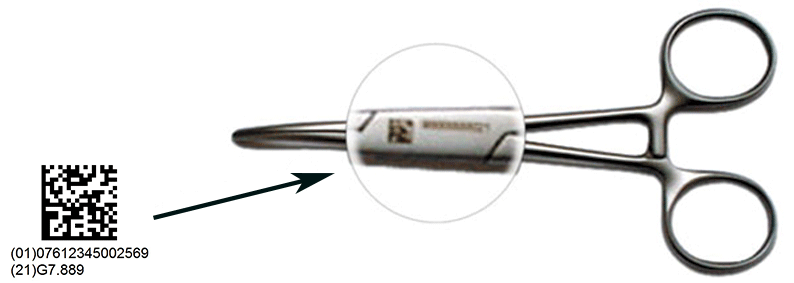
Enhanced traceability from Manufacturers to Patients
Track and Trace solution enables wide-scale benefits starting from the plant level up to the end customer – the patient.
The unique serial number provides tracking of any medical devices and makes the monitoring process easy to be followed. The implementation of traceability solutions will also improve the management of returnable medical devices.
Another benefit of tracking the handling and transportation data (temperature, humidity) of sensitive medical devices, the responsibility of mishandling can be easily determined.
Serialization and aggregation as part of end-to-end traceability will reduce the risks of recalls. by limiting to the exact number of serial numbers rather than whole batches.

