Medical Devices
Traceability has a key role in the monitoring, supervision, and improvement of the overall quality of medical devices and in-vitro devices.
SoftGroup® EUDAMED Gateway
SoftGroup® SaTT EUDAMED Gateway (automatic connection) is a web-based service, part of SoftGroup® SaTT Cloud, that allows an automatic and secured connection, and data exchange between manufacturers and authorized representatives of medical devices, and the European database on medical devices (EUDAMED system).
- Register of medical devices – information about UDI (unique identification code)
- Upload data of certifications, clinical investigations and other documentations
- Upload information about items from the relevant batch
- Verify 2D data matrix
- Check the current status in the EUDAMED
- Update status of items
SoftGroup® SaTT Labeling machine
SoftGroup® SaTT Labeling machine is a specially designed automatic machine for medical devices serialization with the capability to print on labels and verify the 2D Data Matrix and human-readable elements according to EU MDR requirements and GS1 standards. The machine provides second manual verification of the readability of 2D Data Matrix codes via a handheld barcode reader. The machine supports easy changeover of the label templates and different label designs.

- Automated print and verification of 2D Data Matrix codes and human-readable elements on labels according to EU MDR requirements and GS1 standards
- Grades the printed 2D Data Matrix codes
- Second manual verification of the readability of 2D Data Matrix codes with а handheld barcode reader – after applying the label on the medical device
- Easy label template changeovers
- Label designed to survive different storage and extreme temperatures conditions
- Width: 800 mm
- Height: 1700 mm
- Depth: 600 mm
- Gross weight: 170 kg approx.
SoftGroup® SaTT Labeling machine with surface camera
SoftGroup® SaTT Labeling machine with surface camera is a specially designed automatic machine for medical devices labeling and serialization according to EU MDR requirements and GS1 standards. The machine is equipped with additional automatic verification of the readability of 2D Data Matrix codes via а surface camera, grading the quality of printed data after applying the labels. The machine supports easy changeover of the label templates and different label designs.
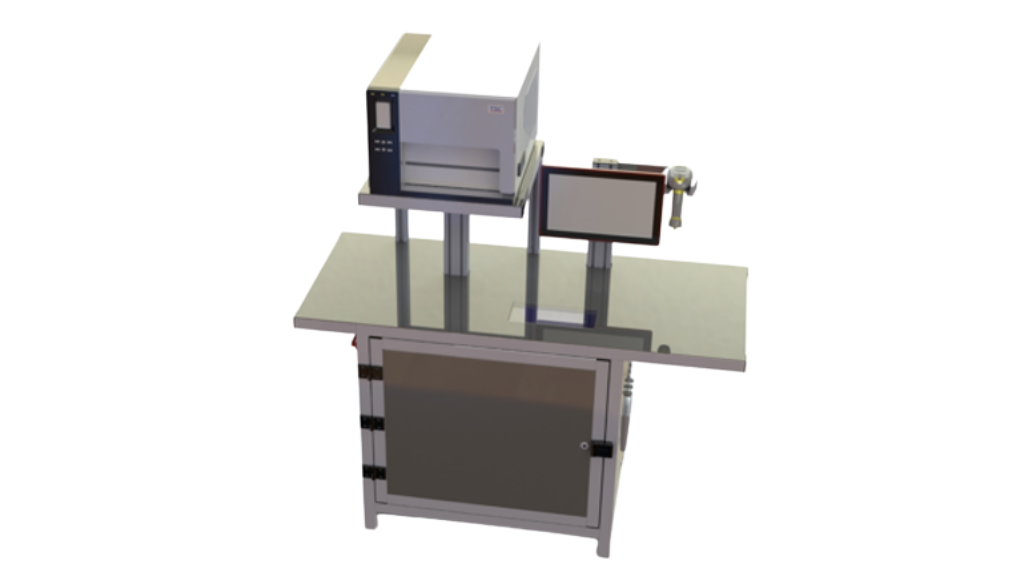
- Automated print and verification of 2D Data Matrix codes and human-readable elements on labels according to EU MDR requirements and GS1 standards
- Grades the printed 2D Data Matrix codes
- Additional automatic verification of the readability of 2D Data Matrix codes with а surface camera- after applying the label on the medical device
- Easy label template changeovers
- Label designed to survive different storage and extreme temperatures conditions
- Width: 1400 mm
- Height: 1600 mm
- Depth: 600 mm
- Gross weight: 200 kg approx.
